Scientific Research of Myko San Preparations


Influence of medicinal mushroom preparations on mouse tumors
Sinisa Ivankovic1, Nevenka Hirsl1, Ivan Jakopovich2, Mislav Jurin1
1Department of Molecular Medicine, Rudjer Boskovic Institute, Zagreb, Croatia
2Dr Myko San – Health from Mushrooms, Zagreb, Croatia
SUMMARY
Extracts from various medicinal mushroom species (Lentinus edodes, Grifola frondosa and Coriolus (=Trametes) versicolor), and in particular their appropriate combinations, are an important precondition for selecting the best among them for the treatment or even prevention of various pathological processes, including cancer. In the present study, the effects of medicinal mushroom preparations on the multiplication of tumor and normal cells, and the survival rate of mice with implanted tumors was investigated. Certain mushroom preparations prevented the incorporation of radioactive 3H-thymidine in the tumor cells of squamous cell carcinoma (SCCVII), fibrosarcoma (FsaR) and melanoma (B16F10) in mice. The incorporation of thymidine only occurs in the DNA in newly produced cells. The effects were dependent on the applied dose of the mushroom preparations, and varied among the individual tumor cell cultures. On the other hand, the incorporation of radioactive 3H-thymidine in normal fibroblasts in the lungs of the Chinese hamster increased, and the effect was generally dose-dependent.
The use of mushrooms preparations in mice with squamous cell carcinoma or fibrosarcoma influences their survival. Thirty days after injection of the squamous cell carcinoma (SCCVII), the survival rate in the control group of mice was 20%, while the survival rate was between 60 and 80% in the groups receiving the mushrooms extracts. On the 36th day, there were no remaining survivors in the control group, while 40-60% of mice in the experimental groups were still alive. Furthermore, on the 36th day after transplantation of tumor cells, the survival rate of mice with fibrosarcoma (FsaR) was 20% in the control group and 60% in the group receiving the mushroom preparations. Of special significance were the findings that the tested mushroom preparations caused selective inhibition of the multiplication of tumor cells in vitro, and a pronounced extension of the life on mice with tumors. These findings, together with the data that these medicinal mushroom extracts are not toxic, deserve special attention.

Running title: mushroom preparations and tumor
The complete text of the article follows below [Download as pdf]
THE INFLUENCE OF MUSHROOM PREPARATIONS ON MOUSE TUMOR
INTRODUCTION
During several thousand years medicinal mushrooms have been used in the treatment of various diseases, including cancer, in traditional medicine (Hartwell, 1971; Hobbs, 1995; Smith et al., 2000). Traditional medicine in many countries as China, Japan, Korea and Russia is well experienced in this field (Hartwell, 1971). Over the last fifty years the scientific interest in active substances in medicinal mushrooms has increased, to clarify the mechanisms of action and to find the most proper way of the application either for the treatment of different diseases (infections, cancer, degenerative processes), or for the prevention of these aberrations (Smith et al., 2000; Smith et al., 2002). This interest is, further, stimulated by the fact that prevention and treatment of cancer and particular virus diseases are not satisfactory in the beginning of the third millennium (Mizuno, 1999; Hara et al., 2003.). Namely, throughout the developed world people are increasingly living longer, but, paradoxically, while life spans are extending, rates of certain human diseases are significantly increasing (Smith et al., 2000). The ability of particular mushroom derived compounds to modulate the human immune response, to lower blood pressure and blood lipid concentration, to inhibit certain tumor growths and microbial activity, and to reduce inflammation is of significant relevance and importance (Wasser and Weiss, 1999).
Extracts from various medicinal mushrooms, and particularly their proper blends, are of significant interest in selecting the best to treat, or even prevent, different pathological aberrations (Smith et al., 2000). In the presented studies possible antitumoros activities of various mushroom extract blends were tested in proper experimental conditions. Namely, effect of mushroom preparations on the in vitro proliferation of tumor and normal cells respectively, as well as the survival rate of the mice with transplanted tumors, were determined.
MATERIALS AND METHODS
Animals
Male mice of C3H/HZgr strain, were used in the experiments. The animals were about 3 months old and weighed 20-23g. They were provided standard diet (Mucedola, Italy) and tap water ad libitum. The light regimen was natural. Animals were treated according to Animal Welfare Regulations.
Mushroom preparations
The commercial blended mushroom preparations Lentifom and Lentram (Dr Myko San – Health from Mushrooms, Zagreb, Croatia) as well as single mushroom preparations of Lentinus edodes (Berk.) Singer (LE), Grifola frondosa (Dicks: Fr.) S.F. Gray (GF) and Coriolus versicolor Quel. (CV), were prepared by the same company for the present study. Generally, 50 g of sporophores were extracted in 1000 ml of boiling water. Extraction was performed for 24 hours by a special approach based on the modified traditional method of making the medicinal decoctions in Far Eastern countries, avoiding any denaturalization, homogenisation or fermentation procedure. The suspensions obtained were forced through a filter press (KSM, Slovenia) to remove insoluble matter, and concentrated to the established basic 4-fold stronger final concentration.
Tumors
C3H male mice were subcutaneously injected into the right thigh with either 100 µl of SCC VII or FsaR tumor cells (5×105 cells per mouse in 100 µl of RPMI) using a tuberculin syringe and a 25-gauge needle. After 7 days, when the tumors were 5-7 mm in diameter, mice were treated with LE, GF, or CV respectively. During 10 consecutive days the dose 0.2g in l ml of saline per mouse was applied, by using a stomach tube. The control group was treated in the same way with 1 ml of saline. There were 10 animals in each group. Animal survival rate was recorded until the day 36 after tumor cell inoculation.
Cell cultures
SCCVII mouse squamous cell carcinoma, FsaR mouse fibrosarcoma, B16F10 mouse melanoma, and Chinese hamster lung fibroblasts (V79) were used in these experiments. Cells were stored in liquid nitrogen. They were defrosted and cultivated in 40 ml culture flasks (Greiner, Germany) together with RPMI-1640 medium (Sigma, USA) and 10% foetal calf serum (FCS) (Sigma, USA). The cultures were kept in an incubator (Heraeus 6000 Germany) at 37 °C in humid atmosphere with 5% CO2 in the air until monolayer cultures covered flasks’ bottoms (usually 24 hours). The cultures were exposed either to the commercial mushroom preparations Lentifom and Lentram diluted to 6.25%, 12.5%, 25% and 50% concentrations respectively, or to single mushroom preparations LE (Lentinus edodes), GF (Grifola frondosa) and CV (Coriolus versicolor) in original concentration or diluted to 25% and 50% respectively. Further, SCCVII and FsaR cells, cultivated for 24 hours in the medium, were used for transplantation in experimental animals.
3H-thymidine incorporation assay
After 24 hours the supernatant was decanted, and 1 ml of trypsin (Sigma, USA) and 5 ml RPMI-1640 supplemented with 10% FCS were added. After a centrifugation at 150 x g for 7 min (Minifuge, Heraeus, Germany), approximately 104 cells/well were incubated further in 200 µl of RPMI 1640 supplemented with 10% FCS for 24 hours in 96-well micro titre plates (Greiner, Germany). Then, cells were labelled with 3H thymidine (1 µCi/well) (Amersham, UK, specific radioactivity 28 Ci/mmol) for 24 hours. Surplus of labelling medium was filtered through “Schleicher” filter (Schuell, Dassel, Germany) in a PHD Cell Harvester (Cambridge Technology Inc. Watertown, USA). Radioactivity of incorporated 3H-thymidine (newly synthesised DNA of proliferating cells) we determined by scintillating b-counter (Wallac 1209 Rackbeta Liquid Scintillation Counter, Pharmacia, Uppsala, Sweden). Results of the 3H thymidine incorporation test were expressed as mean values ± standard deviation (S.D.) of four replicates.
Statistics
The differences between in vitro effects of particular mushroom preparations were statistically evaluated by one-way analysis variance (ANOVA) followed by a post hoc multiple comparison Tukey test. Relative survival time of control and experimental animals was compared using a Fisher`s exact test. These tests were performed using a standard statistical packet, STATISTICA for Windows.
RESULTS
In vitro results
SCCVII, FsaR and B16F10 tumour cells and normal, V 79, cells were cultivated in vitro in the presence of either commercial mushroom blends Lentifom and Lentram or single mushroom preparations Lentinus edodes (LE), Grifola frondosa (GF) and Coriolus versicolor (CV).

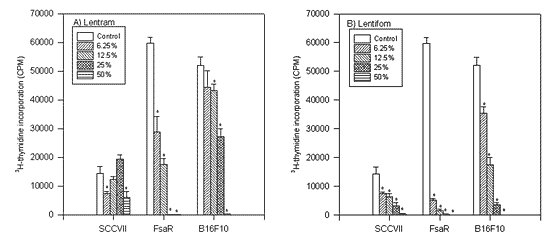
FIGURE 1.
The influence of particular doses of mushroom preparations Lentram and Lentifom on 3H thymidine incorporation in SCCVII, FsaR and B16F10 tumor cells respectively (* p<0.01).
As presented in Figure 1 the application of Lentifom reduced the incorporation of 3H thymidine in SCCVII, FsaR and B16F10 cells, and the effect increased with the dose applied. (p<0.01). Lentram was effective against FsaR and B16F10 cells (p<0.01), although the effect was not so expressed as for Lentifom and significant inhibition of 3H thymidine incorporation in SCCVII cells was visible only in the presence of 6.25% and 50% concentration of Lentram (p<0.01). FsaR cells were the most sensitive to both preparations.

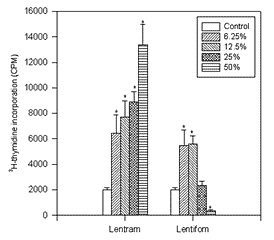
FIGURE 2.
The influence of particular doses of mushroom preparation Lentram and Lentifom on 3H thymidine incorporation in V79 normal cells (* p<0.01).
On the contrary, as presented in Figure 2, 3H thymidine incorporation in normal. V 79 cells was significantly stimulated and increased with the dose when Lentram was applied. (p<0.01). However, the stimulation with Lentifom occurred when small doses (6.25% and 12.5% ) were used (p<0.01) and the highest dose significantly inhibited the incorporation of 3H thymidine (p<0.01).

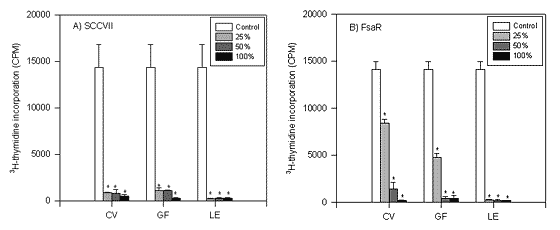
FIGURE 3.
The influence of particular doses of mushroom preparations Coriolus versicolor (CV), Grifola frondosa (GF) and Lentinus edodes (LE), respectively on 3H thymidine incorporation in SCCVII and FsaR cells (*p<0.01).
Experiments were further performed by using single mushroom extracts. As presented in Figure 3, the incorporation of 3H thymidine in tumor cells was inhibited in the presence of CV, GF, or LE. The effect was dose dependent and was different for tumour cell cultures used. Namely, the incorporation of 3H thymidine in SCCVII and FsaR cells was significantly (p<0.01) reduced if any dose of the preparations was applied.
In vivo studies
The mushroom extract dose of 0.2g in l ml of saline per mouse was applied by using a stomach tube daily, during 10 consecutive days, starting at the day 7 after tumor cell transplantation when tumor was palpable. As presented in Figure 4, the application of mushroom preparations LE, GF or CV to SCCVII or FsaR transplanted tumor-bearing mice significantly prolonged their survival.

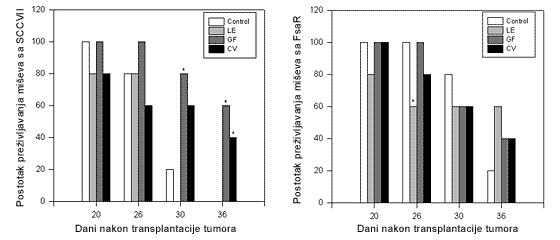
FIGURE 4.
Survival rates of C3H/HZgr mice with tumor following the injection of SCCVII or FsaR cells. After 7 days, when the tumors were 5-7 mm in diameter, mice were treated with preparations of Lentinus edodes (LE), Grifola frondosa (GF) and Coriolus versicolor (CV), respectively. The dose of 0.2g in 1 ml of saline/mouse was applied, by using a stomach tube, during 10 consecutive days. The control group was treated on the same way with 1 ml of saline (*p<0.05).
Namely, on the day 30 after SCCVII tumor cell transplantation 20% of the animals survived in the control group. At the same day there were 60% surviving mice treated with CV (n.s.) and 80% surviving treated with GF (p<0.05). Further, there were no survivors in the control group on the day 36th, but 40% in CV (p<0.05) and 60% in GF (p<0.05) groups were alive. However, LE had no effect on tumor-bearing mice survival rate. It should be noted that CV and GF preparations were very effective against proliferation of SCCVII cells in vitro. Further the survival of FsaR-bearing mice was not different from control values when CV and GF were applied. However, the application of LE influenced the survival of treated mice. Namely, 40% of treated tumor-bearing mice died between the days 20 and 23 following tumor transplantation (p<0.05) when all the control animals were alive. However, at the day 36 there were 60% of LE treated mice alive ( n.s) and only 20% of the controls.
DISCUSSION
Medicinal mushrooms are not “magic bullets” but should be considered as part of an overall strategy for disease prevention and control. The most significant factors of many compounds derived from medicinal mushrooms are their roles as biological response modifiers. While the historical and traditional usage of the medical mushrooms, especially in the Far East, is almost limitless, scientific attention has been given to its ability in immunomodulation and tumor growth inhibition in particular (Jong et al., 1991; Hobbs, 1995; Wasser and Weiss, 1999; Borchers et al., 1999; Wasser, 2002).
It should be mentioned that at times the field of tumour immunology seems mired in unresolved questions. Should the goal of immunization be a CD4+ T cell response or a CD 8+ T cell response? Are the critical cellular elements T cells, dendritic cells, natural killer cells or the others? What is the ideal adjuvant and source of adjuvant? What is the role of endogenous versus exogenous cytokines (Drake and Pardol, 2002). Even a relatively simple matter such as the role of tumour infiltrating lymphocytes remains to be resolved (Ibidem). It should be mentioned that a vast literature, largely based on pathology, has shown that tumour infiltration with lymphocytes bodes an improved prognosis (Eppstein and Fatti, 1976; Nacopoulou et al., 1981; Jass, 1986; Clemente et al., 1996; Ansell et al., 2001). However, recent findings argues strongly to the contrary, accusing these infiltrating lymphocytes and macrophages of promoting metastasis and angiogenesis, and of tipping the immune response from the vigorous cell-mediated immunity expected of a Th1 response to a picture of chronic inflammation more typical of Th2 response (Balkwill and Mantovani, 2001). Thus, the complex events in the organism should be studied and solved. The mechanisms of action of mushroom preparations in tumor bearing animals are not yet well understood but, due to particular experimental models, their beneficial effect could be demonstrated.
It is thought that antitumoros action of medicinal mushrooms is mostly a result of immune system modulation (Borchers et al., 1991). Polysaccharides, including lentinan, were reported as active components in mushrooms. It should be mentioned that natural killer (NK) cells are directly cytotoxic for tumor cells and play a primary role in regulating immune response. A polysaccharide from Grifola frondosa was shown to stimulate NK cells in carcinoma patients and this was expressed for a long period due to the activation by IL 12 released from macrophages (Kodama et al., 2002).
It was reported that lentinan and several other polysaccharides inhibited the growth of sarcoma-180 transplanted subcutaneously in mice (Chihara et al., 1969; Chihara et al., 1970a; Chihara et al., 1970b; Hamuro et al., 1971). Further, a significant growth suppression of human colon carcinoma in the nude mice injected with lymphocytes from the mice pretreated with lentinan was noticed (Ng and Yap, 2002). In addition to the stimulation of the immune reaction against tumor, including mostly T cell stimulation, an attention has focused on the presence of certain substances interacting with the polysaccharides. Three kinds of protein components, different from properdin, increased markedly in mouse serum soon after lentinan administration (Maeda et al., 1974). There is close relationship between the increase of protein components and the antitumor activity of polysaccharides.
Further, it should be noted that several tumor reducing components have been isolated from different mistletoe extracts (Kuttan et al., 1988; Jurin et al., 1993). These extracts contain lectins, viscotoxins and polysaccharides, as three well established components for their clinical application in the therapy of cancer patients in Europe (Hajto et al., 1989). The results in anticancer application of mistletoe point to a direct killing of malignant cells, mostly by cytotoxic lectins and to an induction of mediators of the immune system (Hajto et al., 1989; Jurin et al., 1993).
However, besides polysaccharides action on specific antitumour immune reaction, one should kept in mind the involvement of proteins in the serum. This complex reaction in tumor-bearing animals could be the result of combined effects of polysaccharides and other active components in mushroom preparation (Smith et al., 2000).
Direct antiproliferative action of mushroom preparations used in these studies on tumour cells in vitro, and the observation that their application in tumor bearing mice significantly prolonged survival time, point to beneficial effects in treating cancer.
The finding that mushroom preparations used in these studies were inhibiting the proliferation of tumor but not normal cells is of particular interest. By increasing the doses of Lentram the proliferation of normal V79 cells was considerably stimulated. The stimulation was, further, expressed if Lentifom was used in small doses, whereas higher doses were without the influence or even inhibited cell proliferation in vitro. These data of the stimulatory effect on normal cell proliferation could explain many of beneficial effects (wound healing, immunostimulation) in the organism if mushrooms were used (Jong et al., 1991; Borchers et al., 1999). On the contrary, all mushroom preparations inhibited the proliferation of all tumour cell lines used in these studies and the effect increased with the dose. This selective influence of mushroom preparations should be further investigated. Further, particular mushroom preparations used in these studies significantly prolonged the survival rate of treated tumor-bearing mice. Particular attention should be oriented to a combined therapy by using anticancer drugs and mushroom preparations. These data would be of particular interest for clinical application and deserve further investigation.
Thus, the selective inhibition of tumor cell proliferation observed in vitro and pronounced prolongation of the survival rate of tumor-bearing mice is of particular interest. These findings, together with the data that these compounds are not toxic to the organism, deserve further attention.
REFERENCES
Ansell S.M., Stenson M., Habermann T.M., Jelinek D.F., Witzig T.E. 2001. CD4+ T cell immune response to large B-cell non-Hodkin’s lymphoma predicts patients outcome. J Clin Oncol, 19, 720-726.
Balkwill F., Mantovani A. 2001. Inflammation and cancer: back to Virchow? Lancet, 357, 539-545.
Borchers A.T., Stern J.S., Hackman R.M. 1999. Mushrooms, tumours and immunity. Proc Soc Exp Biol Med ,221, 281-293.
Chihara G., Maeda Y.Y., Hamuro J., Sasaki T., Fukuoka F. 1969. Inhibition of mouse sarcoma 180 by polysaccharides from Lentinus edodes (Berk,) Sing. Nature, 222, 687-688.
Chihara G., Hamuro J., Maeda Y.Y., Arai Y., Fukuoka F. 1970a. Fractionation and purification of the polysaccharides with marked antitumor activity, especially lentinan, from Lentinus edodes (Berk.) Sing. (an edible mushroom). Cancer Res, 30, 2776-2781.
Chihara G., Hamuro J., Maeda Y.Y., Arai Y., Fukuoka F. 1970b. Antitumour polysaccharides derived chemically from natural glucan (Pachyman). Nature, 225, 943-944.
Clemente C.G., Mihm M.C., Bufalino R., Zurrida S., Colini P., Cascinelli N. 1996. Prognostic value of tumour infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer, 77, 1303-1310.
Drake Ch.G., Pardol D.M. 2002. Tumor immunology – towards a paradigm of reciprocal research. SEMIN. Cancer Biol, 12, 73-80.
Epstein N.A., Fatti L.P., 1976. Prostatic carcinoma: some morphological features affecting prognosis. Cancer, 37, 2455-2465.
Hajto T., Hostanska K., .Gabius H.G. 1989. Modulatory potency of the b-galactoside-specific lectin from mistletoe extract (Iscador) on the host defense system in vivo in rabbits and patients. Cancer Res, 49, 4803-4808.
Hamuro J., Yamashita Y., Ohsaka Y., Maeda Y.Y., Chihara G. 1971. Carboxymethylpachymaran, a new water soluble polysaccharide with marked antitumour activity. Nature, 233, 486-487.
Hara M., Hanaoka T., Kobayashi M., Otani T., Adachi H.Y., Montani A., Natsukawa S., Shaura K., Koizumi Y., Kasuga Y., Matsuzawa T., Ikekawa T., Sasaki S., Tsugane S. 2003. Cruciferous vegetables, mushrooms, and gastrointestinal cancer risks in a multicenter, hospital-based case-control study in Japan. Nutr Cancer,2, 138-147.
Hartwell J.L. 1971. Plants used against cancer. Lloydia, 34, 386-437.
Hobbs Ch. 1995. Medicinal Mushroom. An Expoitation of Traditional Healing and Culture. Botanica Press, Santa Cruz. 251 pp.
Jass R. 1986. Lymphocytic infiltration and survival in rectal cancer. J Clin Pathol, 39, 585-589.
Jong S.C., Birmingham J.M., Pai S.H., 1991. Immunomodulatory substances of fungal origin. J Immunol Immunopharmacol, 3, 115-122.
Jurin M., Zarkovic N., Hrzenjak M., Ilix Z. 1993. Antitumorous and immunomodulatory effects of the Viscum album L. preparation Isorel. Oncology, 50, 393-398.
Kodama N., Komuta K., Sakai N., Nanba H. 2002. Effects of D-Fraction, a polysaccharide from Grifola frondosa on tumor growth involve activation of NK cells. Biol Pharm Bull, 12, 1647-1650.
Kuttan G., Vasudevan D.M., Kuttan R. 1988. Isolation and identification of tumor reducing component from mistletoe extract (Iscador). Cancer Lett, 41, 307-314.
Maeda Y.Y., Chihara G., Ishimura K. 1974. Unique increase of serum proteins and action of antitumour polysaccharides. Nature, 252, 250-251.
Mizuno T. 1999. The extraction and development of antitumor-active polysaccharides from medicinal mushrooms in Japan (review). Int J Med Muschrooms, 1, 9-29.
Nacopoulou L., Azaris P., Papacharalampous N., Davaris P. 1981. Prognostic significance of histologic host response in cancer of large bowel. Cancer, 47, 930-936.
Ng M.L., Yap A.T. 2002. Inhibition of human colon carcinoma development by lentinan from shiitake mushrooms (Lentinus edodes). J Altern Complem Med, 5, 581-589.
Smith J.E., Rowan N.J., Tan K.K. 2000. Functional food science and the medicinal mushrooms. Int J Med Mushrooms, 2, 277-285.
Smith J.E., Rowan N.J., Sullivan R. 2002. Medicinal mushrooms: a rapidly developing area of biotechnology for cancer therapy and other bioactivities (review). Biotechnol Lett, 22, 1839-1845.
Wasser S., Weis A.L. 1999. Medicinal properties of substances occuring in higher Basidiomycetes mushrooms: current perspectives (review). Int J Med Mushrooms, 1, 31-62.
Wasser S. 2002. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides (review). Appl Microbiol Biot, 3, 258-274.

